About Us
Our Commitment
Slash 6–12 Months Off FDA/EU Submissions with Our Global Network of Fast-Track Clinical Trial Sites
bioaccess® empowers Medtech and Biopharma innovators to accelerate early feasibility studies and first-in-human trials via Latin America, Eastern Europe, and Australia’s 40% faster approvals, 50% quicker enrollment, and 30% lower costs.
Our Mission
Changing Local Economies
When we and our Medtech or Biopharma clients perform clinical studies in the countries we operate in, the impact on the local economy creates ripples with far-reaching benefits.
These include creating jobs, promoting economic growth, improving healthcare, increasing research and development, and gaining international recognition.
Patient Velocity
Enroll treatment-naive cardiology or neurology cohorts 50% faster than Western sites.
Changing The World
Observing our mission from a bird 's-eye view is ultimately about benefiting humanity as a whole.
Our work with our clients promotes international collaboration and knowledge transfer, leading to better global health outcomes. Most importantly, it drives innovation in Medtech and Biopharma and the next generation of medical innovations.
Why 83% of Startups Choose Latin America, Eastern Europe, and Australia for Early Feasibility Trials
Cost-to-Speed Ratio
$25K/patient savings with FDA-ready data—no rework, no delays.
“bioaccess®’s Colombia trial let us submit PMA data 11 months early. We’re now recruiting patients at 1/3 the US cost.”
Changing Lives
Revolutionizing lives in Latin America, Eastern Europe, and Australia with early access to cutting-edge Medtech and Biopharma innovations, igniting hope and transforming the destinies of those in need and their loved ones.
Seeing the subjects we have recruited smile and be happy again, thanks to successful medical device implants or promising molecules, motivates our team daily.
Regulatory Sprint
Regulatory approval in 6–8 weeks vs. 6–12 months in the US/EU.
Our Story
Pioneering Clinical Shortcuts Since 2010
bioaccess® began with Harvard-trained interventional cardiologist Dr. Pedro Martinez-Clark, an assistant professor at the University of Miami (UM).
In 2008, Dr. Martinez-Clark, Dr. William O'Neill, Dr. Eduardo DeMarchena, and other physician-researchers at UM began collaborating with a research center in Colombia to evaluate the clinical validation of a new cardiac valve that Mitralign (a Boston-area Medtech company) was developing.
A Solution-Oriented Approach
Dr. Pedro Martinez-Clark co-founded bioaccess® after witnessing Boston-based Mitralign lose 9 months to EU delays. By shifting their cardiac valve trial to Colombia, Mitralign achieved:
18-day ethical approval (vs. 6-month EU wait)
11 patients enrolled in 8 weeks (vs. 24-week EU average)
EU submission 14 months ahead of schedule
Mitralign struggled to find a research site in the United States or Europe that could offer fast, cost-effective, and quality first-in-human (FIH) clinical data. Latin America provided the solution, confirming these three requirements.
Dr. Martinez-Clark knew Mitralign wasn’t the only Medtech startup struggling with similar developmental challenges. He realized the bottleneck preventing these companies from advancing their medical devices could be addressed.
The stage was set for creating a gateway that would provide solutions to their needs using Latin America's untapped potential.
In 2010, Dr. Martinez-Clark started a contract research organization (CRO) called Interventional Concepts, dedicated to helping U.S.-based Medtech startups conduct clinical trials in Colombia.
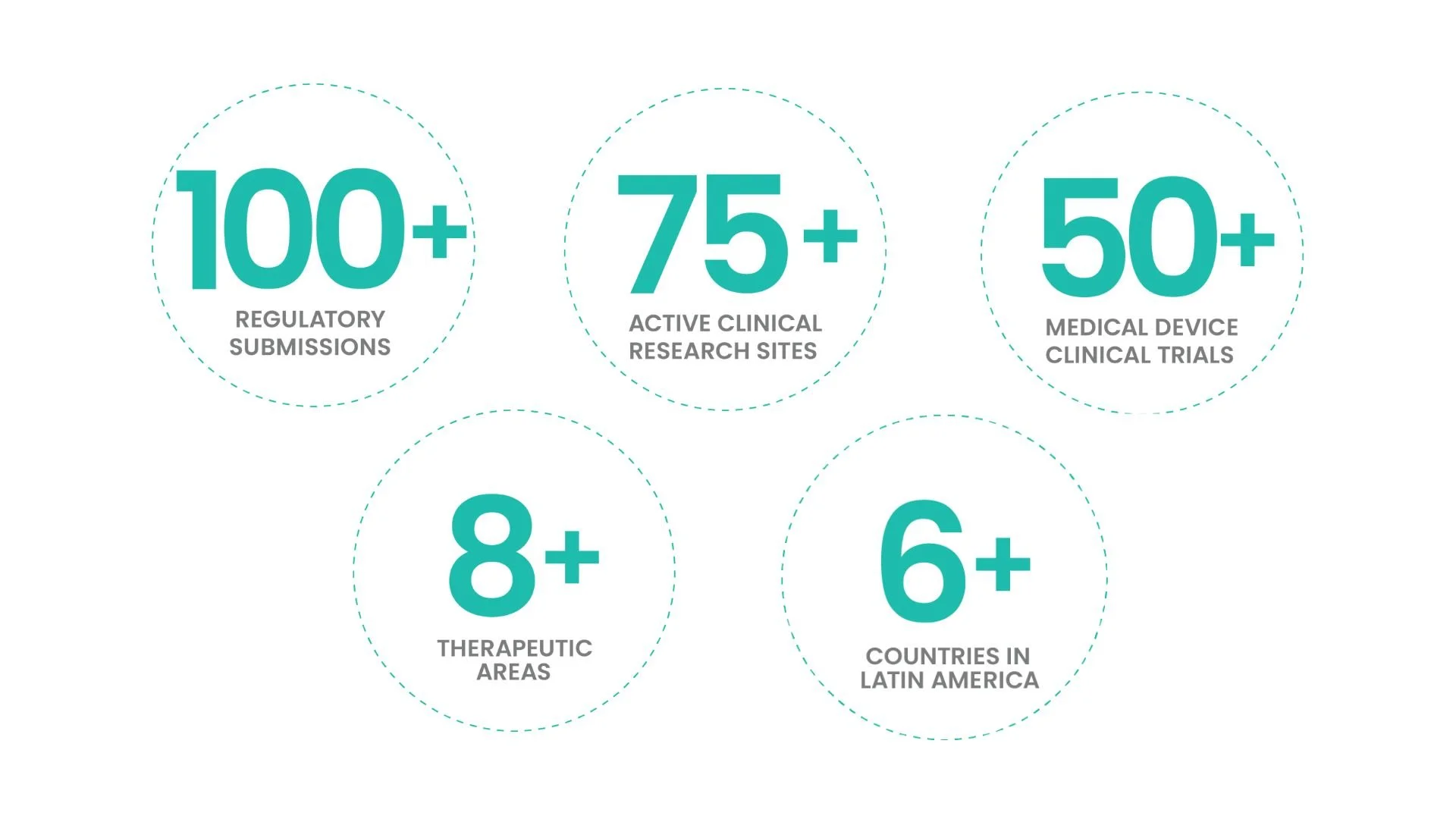
Today, bioaccess® replicates this model across 75+ Latin American, Eastern European, and Australian sites, compressing timelines for 100+ Medtech and Biopharma innovators.
To date, bioaccess® has overseen:
Leadership Team
Julio Martinez-Clark, MBA
-
Julio is the co-founder and CEO of bioaccess®. Julio believes Latin America is an untapped destination for Medtech clinical research. Since 2010, Julio has supported +100 Medtech in operationalizing successful clinical trials in several countries in Latin America. Julio was the chairman of the board of the Association for the Advancement of Clinical Research in Colombia (AVANZAR). Julio writes a column at Med Device Online, where his articles have been "Featured Editorials" on several occasions. Julio hosts the LATAM Medtech Leaders podcast, interviewing Medtech leaders who have succeeded in Latin America.
Julio has a wealth of experience in various fields, including serving as an advisor to the CEO of Amavita Heart and Vascular Health™, the most extensive cardiovascular practice in South Florida, an advisor to government agencies in Colombia looking to position the country as a clinical trial destination, and a mentor to startups at Macondo Labs, a top incubator in Colombia.
In his earlier career, Julio worked at the Johns Hopkins Hospital in Baltimore, MD, as a technology consultant assigned to a project to improve the healthcare system in Trinidad & Tobago. Julio's earlier career includes regional roles for Lucent and Nortel, two leading global telecom and networking companies in the '90s. Julio earned a bachelor's degree in electronics engineering from Saint Thomas University in Bogota, D.C., Colombia, and a master's degree in business administration (M.B.A.) from Western New England University in Springfield, MA. Read more.
Pedro Martinez-Clark, MD, FACC
-
Dr. Martinez-Clark is a world-renowned physician in Miami, Florida. Having earned his medical degree at Colombia’s acclaimed Universidad del Norte in the city of Barranquilla, Pedro traveled to Ohio’s Case Western Reserve University to begin his post-graduate medical training in Internal Medicine. Having excelled and made a name for himself in the northeast, Dr. Martinez-Clark placed into one of the United States’ most competitive Clinical Fellowships in Cardiovascular Disease (Cardiology), at Beth Israel Deaconess Medical Center, Harvard Medical School. At Harvard, he obtained further fellowship training in Interventional Cardiology, Endovascular Therapies and Vascular Medicine. During his stay at Harvard, Dr. Martinez-Clark participated in many clinical trials, research projects and started his involvement in medical device innovation. It would be no surprise that 8 years later, Dr. Martinez-Clark would become recognized, globally for excellence in medical care, research efforts and medical innovation contributions. In addition to caring for patients, Dr. Martinez-Clark works very closely with several public and private organizations with the common goal of improving the healthcare innovation ecosystem in South Florida.
William O'Neill, MD, FACC, MSCAI
-
William O'Neill, M.D., is the Co-founder of bioaccess™. Dr. O'Neill is an internationally recognized leader in interventional cardiology known for pioneering research in new techniques to diagnose and treat heart attacks. He has received numerous honors and awards as an innovator in medicine, a leader in academic and teaching hospitals, and a widely published author.
Dr. O'Neill was involved in seminal trials that spurred important changes in the treatment of heart attacks around the world, including intracoronary streptokinase therapy and emergency angioplasty. He contributed to research demonstrating that heart attack patients have a 90-minute window for treatment before heart muscle begins to die. In 2005, Dr. O'Neill performed the first transvascular aortic valve replacement in the United States as part of a national clinical trial. He also pioneered the mechanical rotational atherectomy technique, using a catheter with a rotating, diamond-tipped burr to carve away and disintegrate plaque that obstructs arteries.
He is a founding member of the American Board of Internal Medicine Interventional Cardiology Board, which certifies all interventional cardiologists. He is the author of more than 300 peer-reviewed articles and abstracts published in medical literature. Dr. O'Neill has written numerous book chapters and edited one of the first textbooks in the field of interventional cardiology.
Dr. O'Neil's honors include Best Doctors in America, America's Top Doctors, and Who's Who in America; Wayne State University School of Medicine Alumni Association's Distinguished Alumni Award (1999); the American Academy of Medical Administrators Nycomed Amersham Award of Excellence (2000) and the TCT (Transcatheter Cardiovascular Therapeutics) Lifetime Achievement Award (2002).
Dr. O'Neill received his medical degree from Wayne State University School of Medicine in 1977. He completed his residency training in internal medicine at the University of Wisconsin at Madison in 1978, and in cardiology at the University of Michigan in 1982.
Monica Mora
-
Monica leads the company's operations, logistics, accounting, human resources, and general corporate and project-related administrative matters. Monica has worked for global companies such as Alcatel-Lucent and Toshiba. Since 2013, Monica has been involved with a myriad of medical device companies to operationalize regulatory, clinical research, and market access/entry strategies in Latin America. Monica has a B.S. degree in computer sciences and graduate studies in business management from Universidad del Norte; the leading university in Colombia's Caribbean coast. Monica is based in Orlando, FL.
John Myklush
-
John Myklusch is a seasoned Exit Planner and Chief Financial Officer with over 20 years of experience in the financial industry. He has a strong track record of successfully guiding businesses through complex exit strategies, including mergers and acquisitions, partnership sales, and private equity investments.
John is a strategic thinker with excellent analytical skills and the ability to identify opportunities for growth and optimization. He is a skilled financial manager with a strong understanding of accounting, budgeting, and forecasting, and has a proven ability to drive financial performance, reduce costs, and improve profitability. He is also adept at managing financial risk and implementing financial controls to protect the organization’s assets.
As a Chief Financial Officer, John is responsible for managing the financial affairs of the organization and ensuring that the company is financially stable and well-positioned for growth. He is skilled at developing and implementing financial plans and policies that align with the long-term goals of the organization.
Jesus Moreno
-
Jesus E. Moreno is a business development director who provides efficient and reliable clinical research services in Latin America. With a passion for driving innovation in the healthcare sector, Jesus leverages his bilingual biomedical engineering background and his understanding of the unique challenges that Medtech startups face to provide valuable support and deliver accelerated clinical study results. Jesus profoundly understands the critical role that reliable and streamlined clinical research plays in the success of Medtech startups. He firmly believes that by offering cost-effective solutions without compromising quality, Medtech startups can achieve their clinical goals faster and make a meaningful impact in the healthcare industry. Jesus is passionate about supporting Medtech startups in their journey towards bringing innovative medical technologies to market. Jesus possesses the technical expertise to understand the complexities of medical devices and technologies. This knowledge enables him to identify tailored solutions that meet the specific needs of Medtech startups. Jesus has a bachelor's degree in biomedical engineering and a master's degree in electronics engineering.
Ana Criado
-
Ana is our regulatory affairs expert and leader. She worked in different executive and leadership roles at Colombia’s regulatory agency —INVIMA— for over five years. She is a biomedical engineering university professor at Universidad Javeriana and Universidad de los Andes, two of the top private universities in Colombia. Ana is an external regulatory consultant for the Colombian operations of global companies such as General Electric, Omron Healthcare, Mindray, and others. She has a degree in chemical pharmacology; a master's degree in health economics & pharmacoeconomics; and certificate degrees in clinical epidemiology, good clinical practices & study monitoring, and pharmacovigilance. Ana is also the founder and CEO of Mahu Pharma, a Colombian company with licenses to cultivate cannabis for medicinal and scientific use and the manufacturing of raw materials for cannabis-based products. Ana is based in Bogota, D.C., Colombia.
Leonardo Rueda
-
With a Bachelor’s degree in Aerospace Engineering and a Master’s in Engineering Management, Leonardo brings over six years of expertise in project management, resource optimization, risk mitigation, and process enhancement.
Leonardo’s career began as a Product Development Engineer in the executive and commercial aviation industry, where he excelled at transforming innovative concepts into practical solutions. Inspired to make a greater impact, he transitioned into clinical research, where he has successfully managed complex projects in both medical device and pharmaceutical trials.
As a Clinical Project Manager, Leonardo demonstrates a steadfast commitment to driving healthcare innovation and improving patient outcomes. His leadership ensures the seamless execution of clinical trials, from strategic planning to successful delivery, aligning with the highest standards of quality and efficiency.
With a unique blend of technical expertise, strategic vision, and a passion for advancing medical research, Leonardo plays a vital role in propelling our mission to redefine clinical research standards and create a lasting impact on global healthcare.
Oswaldo Amaya, MD
-
Oswaldo works as a Director of Editorial and Commercial Research, a Medtech contract research organization (CRO). The company specializes in helping medical device companies conduct clinical trials in Latin America. Oswaldo's clinical expertise and leadership in clinical trials for new drugs and medical devices give him a unique perspective on patient care and the critical role that clinical trials play in advancing medical progress. He has collaborated closely with world-renowned physicians to design and execute clinical studies that advance medical knowledge. As a sub-investigator, he has participated in clinical trials for pharmaceutical companies like Johnson & Johnson, Janssen, INOVIO, and CureVac. He has worked at two of Colombia's most prominent clinical research centers, including the largest research center in the Latin American-Caribbean region. Prior to being a sub-investigator for clinical trials, Oswaldo served as a research associate at the National Institute of Legal Medicine and Forensic Sciences. Oswaldo is passionate about pushing the boundaries of medical science through groundbreaking clinical research and seeks to connect with like-minded professionals who share his dedication. Oswaldo graduated as a medical doctor from Universidad del Norte in Barranquilla, Colombia; one of the top universities in the country.
Katherine Ruiz
-
Katherine is a medical device and in vitro diagnostic regulatory expert and has advised many foreign manufacturers on obtaining market clearance for their innovations in Colombia. Before bioaccess®, Katherine worked at Colombia's regulatory agency, INVIMA, processing import licenses for diagnostic reagents, medical devices, and investigational products for clinical studies. Katherine is an industrial microbiologist from Universidad Javeriana, one of Colombia's top three best universities. She also earned a master's in Quality Management and Integrated Systems from Universidad Santo Tomás in Bogotá, Colombia.
Sergio Alvarado, MD
-
Sergio strongly believes that innovation and medical research are the best ways to significantly impact the medical field. He also recognizes the potential of innovative medical solutions in Latin America, where access to the most recent medical discoveries is an unprecedented opportunity to improve the population’s quality of life.
Early in his career, Sergio became involved in medical research and technology solutions to medical problems. His main area of interest became artificial intelligence, a tool to diagnose medical conditions. Before bioaccess®, Sergio worked in the medical affairs department at Novartis and as a general practitioner for Colsubsudio, one of the top healthcare companies in Colombia. Sergio is constantly broadening his skill set. He has taken courses in epidemiology and public health from the Imperial College London and various soft skills courses as he understands the importance of good communication skills. Sergio speaks fluent English, Spanish, and German and is working on six projects, including degenerative disc disease, venous and artery diseases, and vascular access technologies. Sergio has an M.D. degree from Universidad de Los Andes, one of the top universities in Latin America.
Juan Cuya, MD
-
Juan has experience in regulatory affairs, new product launches, and cost-effectiveness analyses for new drugs in Latin America. His expertise includes systematic literature search, data collection and processing, and development of protocols and scientific articles in different research areas such as epidemiology, neurosciences, anesthesia, and public health. Juan worked as a consultant for different Colombian governmental entities such as the Colombian Ministry of Health, the Institute for Health Technology Assessment (IETS), and international organizations such as PAHO and UNICEF. Juan is a professor at Universidad de Los Andes, teaching young medicine students about creating research projects, the healthcare system, and neglected infectious diseases. At bioaccessTM, Juan's role is to support regulatory submissions for new medical device clinical trials. Juan has a strong interest in research, evidence-based medicine, and the use of new health technologies. Juan holds an M.D. degree from Universidad de Los Andes, one of the top universities in Latin America, and also holds a master's degree in HIV from Universidad Rey Juan Carlos in Spain. Juan is a candidate for a master's degree in epidemiology and public health at Universidad de Los Andes.
Advisory Board
Ron Zelhof
-
With a distinguished career spanning 30 years, Ron Zelhof stands as a beacon in the healthcare sector, recognized for his adept leadership in navigating high-growth healthcare services companies through all stages of development. His unwavering dedication to healthcare innovation and industry progression has been instrumental in the success of private-equity-backed portfolio companies such as Surgery Partners (NASDAQ: SGRY) and AirSculpt Technologies (NASDAQ: AIRS).
Ron has developed unique expertise in executive roles, fostering portfolio companies and spearheading their growth and expansion over an impressive 15-year collaboration with esteemed private equity firms, including H.I.G. Capital, Bain Capital, and Vesey Street Capital Partners.
In Ron's most recent role, he served as the president and chief operations officer of AirSculpt Technologies. His visionary leadership was integral in transforming this founder-led startup into a thriving enterprise. Enacting robust growth plans and driving towards scalability, he navigated the company towards a successful initial public offering (IPO) in November 2021.
Before AirSculpt, Ron was senior vice president of operations for Surgery Partners, a network of ambulatory surgical centers (ASCs). For over a decade, he managed all Florida ASC operations, reinforcing his reputation as a strategic and effective operational leader in the healthcare sector.
Ron's extensive repertoire also encompasses a dynamic and influential tenure at Healthsouth, where he served as vice president of operations. Ron spent over 20 years at Healthsouth, evolving through diverse positions, each marked by his innovative insights and exemplary leadership. His indelible impact at Healthsouth remains a testament to his ability to synergize operational excellence with strategic vision.
In addition to Ron's accomplished career, he currently lends his vast experience and industry expertise to bioaccess™ as an advisory board member. Ron holds a bachelor's degree in education and another in physical therapy from the University of Miami. Ron's robust academic foundation and practical insights bolster his innovative strategies and business acumen.
Michael Alvarez
-
Michael has been building strategic partnerships between industry, government, academia, and non-profits for more than twenty-five years. Early in his career he was a management consultant with Accenture in NYC, focused on corporate biopharma and energy clients.
He later founded and directed centers for entrepreneurship, industry engagement, and workforce development at several premier institutions highly regarded across the globe including Stanford University, Columbia University, UC San Francisco, and MD Anderson Cancer Center.
He is widely sought for input and expert opinion on human and planetary health, technology innovation, life science training, business strategy, and the future of work in emerging and evolving industry sectors. He is an established author and has addressed these topics in key venues such as Nature, Forbes, US News and World Report, and the National Public Radio.
He continues to advance bioscience and global climate solutions through his role as program director for QB3 at UCSC, and as an investor and an advisor for life science companies. He formerly served as chair and as a board member for many years for the Northern California SF based Bay Bio Institute (successfully expanded now into the larger state-wide enterprise known as California Life Sciences).
He continues to serve his heritage as a first-generation born US-Latino supporting the Hispanic community abroad as a member of the Hospital de La Familia Foundation board, improving healthcare in Guatemala through hospital, eye clinic, and nutrition center operations; and locally on the Hispanic Foundation of Silicon Valley board, where he chairs the Nominations and Governance committee. Michael holds bachelors and masters degrees from Boston College, and certificates in Intellectual Property Management and Bio-Executive Education from the University of Washington and the UC Berkeley Haas School of Business respectively.