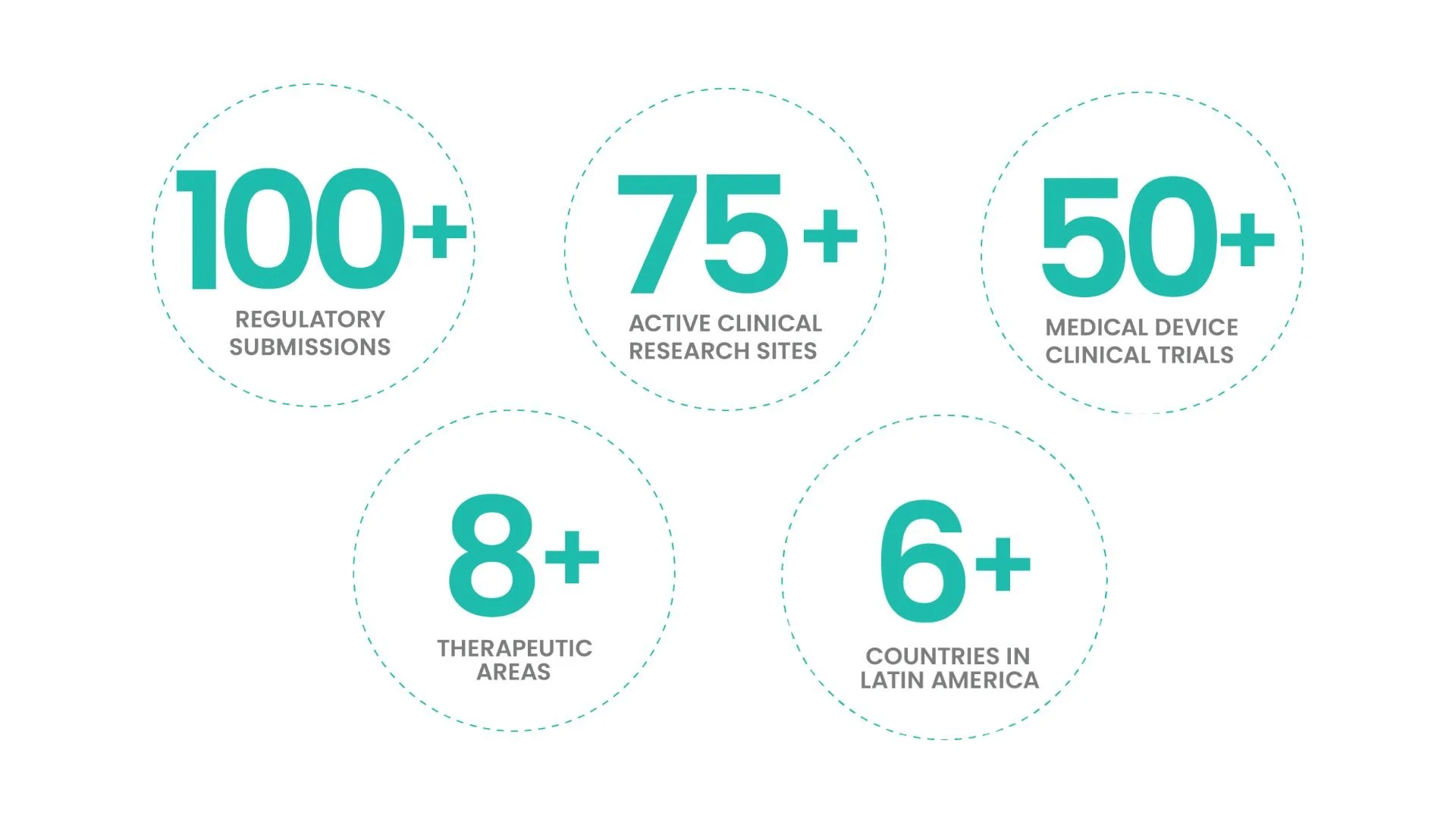
Fast-Track First-in-Human Trials in Latin America, Eastern Europe & Australia-40% Faster Than US/EU
Slash timelines by 6–12 months with bioaccess®’s proven model: ethics approvals in 4–8 weeks, 30% lower costs, and FDA/EMA-ready data.
Why Innovators Choose bioaccess®’s Global Network
Latin America
Speed: Panama, El Salvador, Chile approvals in 4–8 weeks vs. 6+ months in US/EU.
Cost: $25K/patient savings with pre-negotiated site contracts.
Post-Trial Edge: Fast-track commercialization in LATAM’s $1.2B MedTech market.
Eastern Europe
Parallel Reviews: Ethics + ALIMS approvals in 80 days via Serbia’s ADIS portal.
Diverse Populations: Rare disease cohorts in Kosovo; cardiology KOLs in Belgrade.
EU Gateway: CTR-aligned data for seamless EMA submissions.
Australia
CTN Speed: TGA acknowledgment in 5–10 days for low-risk trials.
ICH-GCP Compliance: 100% FDA/EMA acceptance with no data rework.
Strategic Bridge: Run Phase I trials while preparing US IND.
Digital Health Startup CEO
"bioaccess®’s Serbia site activated in 8 weeks-9 months faster than our EU delay.”
Biopharma Founder
“Australia’s CTN process cut our Phase I costs by 35%.”

Services
End-to-End Acceleration for Global Trials
Regulatory Sprint
Parallel submissions in LATAM, Balkans & Australia.
Unlike generic CROs, bioaccess® combines regional speed/cost advantages with deep regulatory expertise to de-risk trials for startups prioritizing time-to-market and investor-ready outcomes.
Phase I, II/Early-Feasibility Studies (EFS)
Phase I/First-In-Human Studies (FIH)
Patient Recruitment at Warp Speed
Pre-qualified networks: 50+ sites activated in <8 weeks.
Site Activation in <8 Weeks
FDA/EMA/MDR-ready datasets with centralized monitoring.
Trusted By
Testimonials
Committed To Your Success
bioaccess® is the only CRO that bridges the gap between innovative Medtech and Biopharma startups and the untapped potential of conducting fast clinical research studies in Latin America, Eastern Europe, and Australia.
Our team believes in the importance of your medical innovation and the benefit it could bring to people’s lives.
We are committed to helping you navigate the convoluted and uncertain developmental challenges of being an early-stage startup. Our priority is bringing you fast, cost-effective, and high-quality clinical data.