
News


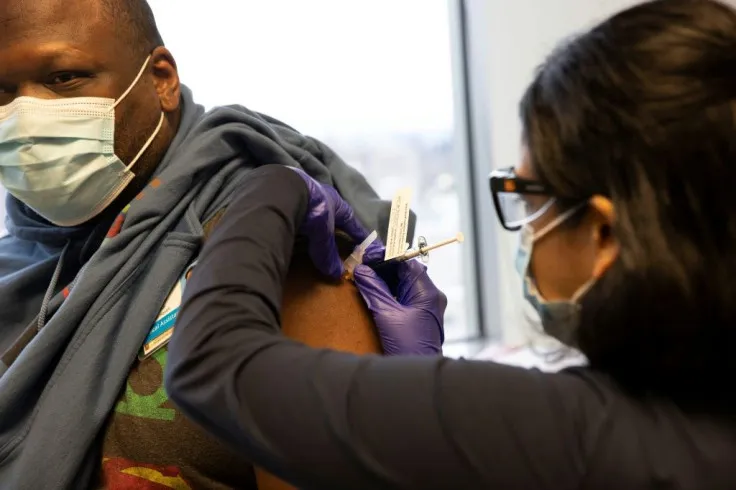
How Medtech Companies are Unlocking the Potential of Latin America in Clinical Research
Medtech startups' challenges in the medical device industry in the US are multifaceted, ranging from regulatory hurdles to limited financial resources and prolonged subject recruitment timelines. US Medtech companies face professionalism, language barriers, fragmentation of resources, and lack of CRO corporate structures in Latin America. This impedes Latin American hospitals from having seamless communication and collaboration with American clinical trial clients, particularly in the medical device first-in-human clinical trial industry. These factors underscore the urgent need for a solution-driven approach to bridge the gap between innovation and execution in Latin America.

eyeFlow, Inc. Obtains Colombia Approval for its Pilot Clinical Study
On February 14, 2024, INVIMA, Colombia's regulatory agency, approved a pilot study by eyeFlow, Inc. at one research center in Barranquilla. This pilot clinical trial aims to recruit 60 subjects in Colombia and will last approximately 18 months.

i-Lumen Scientific, Inc. Obtains Colombia Approval for its i-Sight 2 Clinical Study
On January 17, 2024, Colombia's regulatory agency, INVIMA, approved i-Lumen Scientific, Inc.'s i-Sight 2 study at two research centers, one in Medellín and another in Cali. The i-Sight clinical trial seeks to recruit up to 75 subjects in Colombia and will last about 27 months.

3ive Labs Obtains Colombia Approval for its BIPASS-AKI 2 Study
On November 15, 2023, INVIMA, Colombia's regulatory agency, approved the BIPASS-AKI-2 early feasibility study by 3ive Labs, LLC (aka “Roivios™), with its JuxtaFlow ® (RAD) renal assist device at two research centers, one in Barranquilla, and the other one in Bucaramanga. This early feasibility study aims to recruit up to 40 subjects in Colombia and will last approximately 30 months.

Imperative Care Obtains Colombia Approval for its ADVANCE First-In-Human study
On September 28, 2023, INVIMA, the regulatory agency of Colombia, approved Imperative Care, Inc.'s ADVANCE first-in-human study, which will be conducted at one research center in Cartagena. The ADVANCE study aims to recruit up to 15 subjects from Colombia and will last for approximately five years.

Cook Medical Treats First Patient in First-In-Human Clinical Trial for Venous Valve
Cook Medical announces the first patient treated in a clinical study to evaluate a new venous valve for treating chronic venous insufficiency. The patient was treated by Dr. Mauricio Alviar, vascular surgeon and principal investigator of Clinica de la Costa in Barranquilla, Colombia.

Hancock Jaffe Principal Investigator Dr. Jorge Hernando Ulloa Presents VenoValve One Year First-In-Human Data at Charing Cross International Symposium
IRVINE, CA / April 23, 2021 / Hancock Jaffe Laboratories, Inc. (NASDAQ:HJLI), a developer of medical devices that restore cardiac and vascular health, today announced that Dr. Jorge Hernando Ulloa, the Principal Investigator for HJLI’s first-in-human VenoValve® study in Bogota, Colombia, presented one year data this past week at the Charing Cross International Symposium. The Charing Cross International Symposium is the longest-running vascular and endovascular global symposium in Europe and is attended by the world’s leading experts in vascular and endovascular medicine.
ReGelTec Successfully Treats First Eleven Chronic Low Back Pain Patients with HYDRAFIL™ in Colombia
ReGelTec, Inc., a medical device company developing a percutaneous treatment for chronic low back pain, announced today that eleven patients with degenerative disc disease have been enrolled in the Company’s Early Feasibility Study in Barranquilla, Colombia. The procedures were proctored remotely via Zoom, and all eleven patients were successfully treated with HYDRAFIL™. This patented hydrogel is melted before injection into the nucleus of a degenerated disc via a 17-gauge needle.

Breakthrough Gene Therapy Clinical Trial is the World's First That Aims to Reverse 20 Years of Aging in Humans
MANHATTAN, Kan., Nov. 21, 2019 /PRNewswire/ -- Libella Gene Therapeutics, LLC ("Libella") announces an institutional review board (IRB)-approved pay-to-play clinical trial in Colombia (South America) using gene therapy that aims to treat and ultimately cure aging. This could lead to Libella offering the world's only treatment to cure and reverse aging by 20 years.
